Science has really benefitted me through these years, and that it had developed an interest into me. This year, we have learnt on various topics, such as chemistry-atom structure,reactions , physic-reflection and refraction,colours of light, lenses, and biology-ecology,sexual reproduction.
It was fairly easy to learn these topics , although I still faced various difficulties at the start. When we first started on these topics, many questions were in my head. Like, why did that happen? how does it happen? However, this was quickly resolved as I had asked our teacher, and also conducted my own research. The hardest topic I found was reflection and refraction. This was because at the start, I did not grasp the concept, and had to inquire a lot about it to understand the reason behind it. However, after that, it was fairly simple to understand the other related topics on colours of light and lenses.
Other than that, biology was a new topic that we were taught on. Ecology was fairly simple, but there was a lot of information to learn about. Therefore, I feel that my progress is still decent, and that my growth development in science is still ok.
Friday, September 9, 2011
Wednesday, September 7, 2011
Term test reuslts and reflection
This term, I did quite well for my science and have managed to acheive an A1 for science. The topics tested were colours of light, lenses, and ecology. This was fairly easy once I understood the concept, and after revising, the test was managable to complete. I have learnt a lot about these topics, and am very interested after it was taught. I also conducted my own research on such topics to understand further about it. However, I should not take science as a simple subject and be arrogant about it, and that I would definately continue this good progress. My goal now is to maintain this A1 result in EOY, and my progress would be to start my revision now, during the September holiday.
Thursday, September 1, 2011
Colours of light-Wavelength
When light travels through a prism, it would disperse into the different colours of light. Red, orange, yellow, green, blue, indigo, violet. The white light would disperse into a visible spectrum of light, containing the colours of the rainbow. This is because of the refraction of the lights. The lights are refracted at different angles, resulting in the spectrum.
This made me wonder, why would it be refracted at different angles?
This is due to the different wavelengths. As red has a greater wavelength of 700mm, it would travel faster than the other colours, and thus be refracted least. While indigo has a wavelength of 400mm, it travels the slowest, and thus be refracted more. This would result in the dispersion of light. As light passes the different mediums of air to glass, the reduce in speed causes refraction. At different speeds, the different colour lights would be refracted at different angles, resulting in a visible spectrum.
Dispersion of white light
Different wavelengths resulting in the angle of refraction
Saturday, August 27, 2011
Application of TIR-total internal reflection
Through our science lessons, we got to learn about refraction and total internal reflection, which takes place because 1)the incident angle is greater than critical angle 2)the incident ray is in the optically denser medium.
The critical angle is the incident angle whereby the angle of refraction is 90 degrees
After learning this, I conducted an ACE assignment on the application of total internal reflection.
1)Optical fibres
Optical fibres make use of TIR to trasmit data from one end to the other. It is made of pure glass, and transmits light from one end to the other through TIR within the fibre. Therefore, it would have the advantage of no electromagnetic interference, and therefore lesser loss of signal. It is also thin and flexible.
2)Medical equipment
Such equipment are like endoscope, colonoscope, otoscope and amnioscope. These equipment make use of TIR examine the internal organs in our body.For example, in the endoscope, it is used to examine the lungs. Hence, light is required to see. Light is transmitted through optical fibres, which provide the light source and enables the doctor to examine the lungs.
The critical angle is the incident angle whereby the angle of refraction is 90 degrees
After learning this, I conducted an ACE assignment on the application of total internal reflection.
1)Optical fibres
Optical fibres make use of TIR to trasmit data from one end to the other. It is made of pure glass, and transmits light from one end to the other through TIR within the fibre. Therefore, it would have the advantage of no electromagnetic interference, and therefore lesser loss of signal. It is also thin and flexible.
2)Medical equipment
Such equipment are like endoscope, colonoscope, otoscope and amnioscope. These equipment make use of TIR examine the internal organs in our body.For example, in the endoscope, it is used to examine the lungs. Hence, light is required to see. Light is transmitted through optical fibres, which provide the light source and enables the doctor to examine the lungs.
Wednesday, June 22, 2011
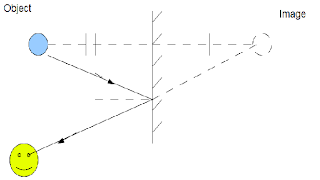
Contruction Ray Diagrams
How to construct a ray diagram on a plane mirror.
This is a sum-up of how to do so:
1)Locate the image point. One of the image property of a plane mirror is that the distance between the image and the mirror is equal to the distance between the object and the mirror. Therefore, we would be able to locate the image point.
2)Draw a light ray from the eye to the image. When we look into a mirror, we would see the image. Therefore, we can draw a straight line from our eye to the image point.(Use dotted lines behind the mirror)
3)Draw a line from the object to the mirror point where the light ray had intercepted.
This is a simple way of constructing a ray diagram.
This is a sum-up of how to do so:
1)Locate the image point. One of the image property of a plane mirror is that the distance between the image and the mirror is equal to the distance between the object and the mirror. Therefore, we would be able to locate the image point.
2)Draw a light ray from the eye to the image. When we look into a mirror, we would see the image. Therefore, we can draw a straight line from our eye to the image point.(Use dotted lines behind the mirror)
3)Draw a line from the object to the mirror point where the light ray had intercepted.
This is a simple way of constructing a ray diagram.
Monday, June 20, 2011
Reflection and Refraction
This topic was rather tough at first, as I did not understand the reason for it, and how to constrcut the diagrams. However, after the first week of lessons, I managed to understand, and was more interested in this topic. To learn this topic, we went through lessons and experiments. The experiments really benefitted us as we got to understand reflection and refraction through activities, which really allowed us to understand better.
Reflection
-All surfaces reflect light
-Definitions
Normal:An imaginary line perpendicular to surface
Incident ray:Ray sriking surface of plane
Reflected ray:Ray reflected of surface of plane
-Rules of reflection
1st rule: The incident ray, normal and reflected ray all lies on the same plane
2nd rule:Angle of incidence=Angle of reflection
-Characteristics of a image formed on a plane mirror
Upright,Virtual,Same size as object, laterally inverted, same distance as object
A virtual image is one that cannot be formed on a screen as actual light rays does not meet at the image point. Instead, the light rays diverge to form the vitual image.
A real image is one that can form on a screen as actual light rays meet at image point.
Reflection
-All surfaces reflect light
-Definitions
Normal:An imaginary line perpendicular to surface
Incident ray:Ray sriking surface of plane
Reflected ray:Ray reflected of surface of plane
-Rules of reflection
1st rule: The incident ray, normal and reflected ray all lies on the same plane
2nd rule:Angle of incidence=Angle of reflection
-Characteristics of a image formed on a plane mirror
Upright,Virtual,Same size as object, laterally inverted, same distance as object
A virtual image is one that cannot be formed on a screen as actual light rays does not meet at the image point. Instead, the light rays diverge to form the vitual image.
A real image is one that can form on a screen as actual light rays meet at image point.
Saturday, June 4, 2011
Chemical bonding
In Sec 2, we have learnt about 2 different forms of chemical bonding. These are ionic bonding and covalent bonding.
Ionic Bonding
Ionic bonding takes place between two or more elements whereby electrons are transferred from one atom to another. This would form ions, and an ionic compound. This takes place between metal and non-metal elements. Examples of ionic compounds are NaCl-Sodium Chloride, CaCl2-Calcium Chloride.
Convalent Bonding
Covalent bonding takes place between metal to metal, or non-metal to non-metal elements. It is a process whereby the electrons are shared between the two elements. For example, Oxygen has an electron configuration of 2.6 . Therefore, it requires 2 more electrons to complete its valence shell and be a stable atom. As such, an oxygen atom would share two electrons with another oxygen atom, and would form the oxygen molecule, O2. Other examples are H2-Hydrogen molecule and H2O-water
Ionic Bonding
Ionic bonding takes place between two or more elements whereby electrons are transferred from one atom to another. This would form ions, and an ionic compound. This takes place between metal and non-metal elements. Examples of ionic compounds are NaCl-Sodium Chloride, CaCl2-Calcium Chloride.
Convalent Bonding
Covalent bonding takes place between metal to metal, or non-metal to non-metal elements. It is a process whereby the electrons are shared between the two elements. For example, Oxygen has an electron configuration of 2.6 . Therefore, it requires 2 more electrons to complete its valence shell and be a stable atom. As such, an oxygen atom would share two electrons with another oxygen atom, and would form the oxygen molecule, O2. Other examples are H2-Hydrogen molecule and H2O-water
Friday, March 18, 2011
Sabbatical-Science Eureka
For Term 1 sabbaticals, I attended this course, Science Eureka. This was because I was interested in Science, and found this course very meaningful for me. We conducted experiments of many kinds, physics and chemistry. To me, I prefered chemistry, and the experiments were also very fun. Here are a few...
1) Making snow
We used sodium polycrylate, a chemical able to absorb 800 times it mass in water, and could form beads which resemble snow. This was a very quick and easy process, and was very fun as we did it, and we got to learn a lot from it. We also got to keep the snow afterwards.
2) Artificial Blood
There are many uses for artificial blood, as seen in movies and tv shows. However, how do they make the "blood". Well, hollywood does it this way. It is a combination of Iron(|||)Nitrate and Sodium Thiocyanate. This forms a colour that is nearly identical to blood, and makes it seem very guesome. It was also a fun process.
3)Apple juice
To produce more apple juice (although may not be drinkable), there is this enzyme pestinase. This substance is able to break down pectin, which is a substrate found in the cell wall of plants. This would then release more juice.
The sabbatical is very enjoyable, as well as educational. We conducted many more experiments, and these are just only a few. I really enjoyed it, and am very interested in such courses in the future.
Thursday, March 10, 2011
Importance of Science
Science is really important in our lives. This is because of many reasons. Just stating a few, science can allow us to understand our universe, understand our history, and maintain our earth's sustanability for living, and also benefit us in our daily lives.
Through new discoveries in technology, man has been able to send up satellites into space, and also allowed man to explore space. Through this, it could also give rise to commercial space travel. We can also observe other planets, and conduct so much research on our universe, so that we could understand our planet and world better.
We can also discover our history. Through science technology, we can discover ancient artifacts such as vases from ancient China, our fossils of dinosaurs living more than 5 billion years ago. Through this, we can understand more of our past and about man's evolution.
Other than that, science also helps us to maintain our earth's sustanability. We need resourses for living, such as food and water. Through technology, such as NEWater and distillation plants, we would be able to produce our own drinkable water. Other than that, conserving energy devices such as solar panels are invented because of the development of science. This helps us to conserve earth's scarce resource, and also protect our environment.
Other science applications are those used to catch criminals. Such as DNA scanner, which are able to help police discover the offender. Or our living environment, such as televisions, lighting. All this technology is based on science discoveries, and which is why science is important.
Through new discoveries in technology, man has been able to send up satellites into space, and also allowed man to explore space. Through this, it could also give rise to commercial space travel. We can also observe other planets, and conduct so much research on our universe, so that we could understand our planet and world better.
We can also discover our history. Through science technology, we can discover ancient artifacts such as vases from ancient China, our fossils of dinosaurs living more than 5 billion years ago. Through this, we can understand more of our past and about man's evolution.
Other than that, science also helps us to maintain our earth's sustanability. We need resourses for living, such as food and water. Through technology, such as NEWater and distillation plants, we would be able to produce our own drinkable water. Other than that, conserving energy devices such as solar panels are invented because of the development of science. This helps us to conserve earth's scarce resource, and also protect our environment.
Other science applications are those used to catch criminals. Such as DNA scanner, which are able to help police discover the offender. Or our living environment, such as televisions, lighting. All this technology is based on science discoveries, and which is why science is important.
Wednesday, March 2, 2011
Science Lab Lessons
Every week, our class has one science lab lesson, where we visit the science lab to conduct various experiments. The experiments were very helpful in our learning, as we got to understand more about what we learn. Such experiments we conducted was on chemistry. My favourites was when had experiments on acid reactions. For example, what happens when acid reactions with carbonate.
Acid+Metal-->Salt+Hydrogen
Eg.Hydrochloric Acid+Iron-->Iron(||)Chloride+Hydrogen
Acid+Carbonate-->Salt+Water+Carbon Dioxide
Eg.Sodium Carbonate+Nitric Acid-->Sodium Nitrate+Water+Carbon Dioxide
Acid+Bases-->Salt+Water
Eg.Nitric Acid+Potassium Hydroxide-->Potassium Nitrate+Water
From the experiment, I also found out that some common salts were nitrates, sulfate, and chloride. And that common acids were hydrochloric acid, nitric acid, and sulfuric acid. We also found out about the application of these acids. We could conduct the experiments ourself, and thus develop a greater understanding on it, rather than listening to the teacher explain.
Thus, I feel that Science Lab lessons are very meaningful and helpful.
Subscribe to:
Comments (Atom)