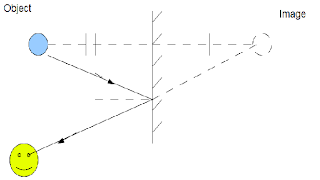
How to construct a ray diagram on a plane mirror.
This is a sum-up of how to do so:
1)Locate the image point. One of the image property of a plane mirror is that the distance between the image and the mirror is equal to the distance between the object and the mirror. Therefore, we would be able to locate the image point.
2)Draw a light ray from the eye to the image. When we look into a mirror, we would see the image. Therefore, we can draw a straight line from our eye to the image point.(Use dotted lines behind the mirror)
3)Draw a line from the object to the mirror point where the light ray had intercepted.
This is a simple way of constructing a ray diagram.
Wednesday, June 22, 2011
Monday, June 20, 2011
Reflection and Refraction
This topic was rather tough at first, as I did not understand the reason for it, and how to constrcut the diagrams. However, after the first week of lessons, I managed to understand, and was more interested in this topic. To learn this topic, we went through lessons and experiments. The experiments really benefitted us as we got to understand reflection and refraction through activities, which really allowed us to understand better.
Reflection
-All surfaces reflect light
-Definitions
Normal:An imaginary line perpendicular to surface
Incident ray:Ray sriking surface of plane
Reflected ray:Ray reflected of surface of plane
-Rules of reflection
1st rule: The incident ray, normal and reflected ray all lies on the same plane
2nd rule:Angle of incidence=Angle of reflection
-Characteristics of a image formed on a plane mirror
Upright,Virtual,Same size as object, laterally inverted, same distance as object
A virtual image is one that cannot be formed on a screen as actual light rays does not meet at the image point. Instead, the light rays diverge to form the vitual image.
A real image is one that can form on a screen as actual light rays meet at image point.
Reflection
-All surfaces reflect light
-Definitions
Normal:An imaginary line perpendicular to surface
Incident ray:Ray sriking surface of plane
Reflected ray:Ray reflected of surface of plane
-Rules of reflection
1st rule: The incident ray, normal and reflected ray all lies on the same plane
2nd rule:Angle of incidence=Angle of reflection
-Characteristics of a image formed on a plane mirror
Upright,Virtual,Same size as object, laterally inverted, same distance as object
A virtual image is one that cannot be formed on a screen as actual light rays does not meet at the image point. Instead, the light rays diverge to form the vitual image.
A real image is one that can form on a screen as actual light rays meet at image point.
Saturday, June 4, 2011
Chemical bonding
In Sec 2, we have learnt about 2 different forms of chemical bonding. These are ionic bonding and covalent bonding.
Ionic Bonding
Ionic bonding takes place between two or more elements whereby electrons are transferred from one atom to another. This would form ions, and an ionic compound. This takes place between metal and non-metal elements. Examples of ionic compounds are NaCl-Sodium Chloride, CaCl2-Calcium Chloride.
Convalent Bonding
Covalent bonding takes place between metal to metal, or non-metal to non-metal elements. It is a process whereby the electrons are shared between the two elements. For example, Oxygen has an electron configuration of 2.6 . Therefore, it requires 2 more electrons to complete its valence shell and be a stable atom. As such, an oxygen atom would share two electrons with another oxygen atom, and would form the oxygen molecule, O2. Other examples are H2-Hydrogen molecule and H2O-water
Ionic Bonding
Ionic bonding takes place between two or more elements whereby electrons are transferred from one atom to another. This would form ions, and an ionic compound. This takes place between metal and non-metal elements. Examples of ionic compounds are NaCl-Sodium Chloride, CaCl2-Calcium Chloride.
Convalent Bonding
Covalent bonding takes place between metal to metal, or non-metal to non-metal elements. It is a process whereby the electrons are shared between the two elements. For example, Oxygen has an electron configuration of 2.6 . Therefore, it requires 2 more electrons to complete its valence shell and be a stable atom. As such, an oxygen atom would share two electrons with another oxygen atom, and would form the oxygen molecule, O2. Other examples are H2-Hydrogen molecule and H2O-water
Subscribe to:
Comments (Atom)